High quality products to support Pathologists and Biological and Environmental Scientists
Coronavirus (COVID-19, Strain 2019-nCoV)
$1,321.04Product Features
- Exceptional value for money
- Rapid detection of 2019-nCoV
- Positive copy number standard curve for quantification
- Highly specific detection profile
- High priming efficiency
- Broad dynamic detection range (>6 logs)
- Sensitive to < 100 copies of target
- Accurate controls to confirm findings
COVID-19 Real-Time PCR assay EUA (USA)
$1,321.04Product Features
- FOR US ONLY
- Rapid detection and exclusive to the COVID-19 strain
- Does not detect other related coronavirus strains
- High priming efficiency
- Accurate controls to confirm extraction, and assay validity
- Lyophilised components for ambient shipping
- Highly specific detection profile
This test has been authorized by FDA under an EUA for use by authorized laboratories.
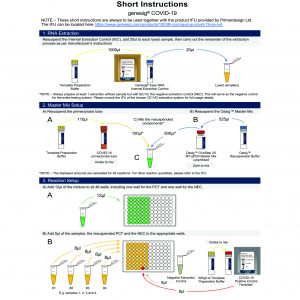
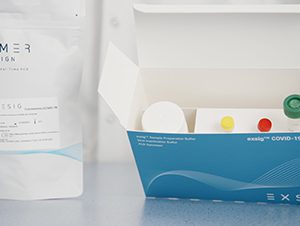
exsig® COVID-19 Direct
$2,103.08A total workflow solution with all reagents and components provided from start to finish.
- Total workflow solution
- All reagents and components provided from start to finish
- Rapid protocol with fewer handling steps
- For use with dry swabs
- Viral inactivation; Preparation, Amplification, Diagnosis
- Total chemistry optimisation
- Low shipping costs with ambient shipping
- Includes our genesig® Real-Time PCR Coronavirus COVID-19 (CE IVD)
SNPsig® COVID-19 (20I/501Y.V1+E484K) Identification Kit
$1,500.00- For the detection of the SARS-CoV-2 variants with the 20I/501Y.V1, VOC-21FEB-02 and variants carrying the E484K mutation
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix
SNPsig® COVID-19 (B.1.1.519)
$1,500.00- For the detection of the SARS-CoV-2 variants with the B.1.1.519
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix
SNPsig® EscapePLEX™ SARS-CoV-2
$1,505.00Product Features
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions
SNPsig® SARS-CoV-2 (20B/484K) Identification Kit
$1,500.00- Detection of the SARS-CoV-2 variants with the 20B/S.484K mutation, also known as P2
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix
SNPsig® SARS-CoV-2 (20H/501Y.V2) Identification Kit
$1,500.00- For the detection of the SARS-CoV-2 (20H/501Y.V2 South Africa)
- Rapid detection of specific detection profiles
- High priming efficiency
- Sensitive to < 100 copies of target
- Positive copy number standard curve for quantification
- Accurate controls to confirm findings
- 96 reactions, includes master mix