High quality products to support Pathologists and Biological and Environmental Scientists
Fentanyl (FYL) Detection Kit (Rapid Lab)
$200.00
- Rapid screening test for the qualitative detection of Fentanyl (FYL)
- Format: 10 tests
- Run Time: 10 Minutes
For professional use only, not for self-testing.
Mpox Virus (MPV) Antigen test
$200.00- 10 tests per kit.
- Monkeypox Virus Antigen Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of monkeypox virus antigen in body fluid samples.
For professional medical institutions use only, not for self-testing.
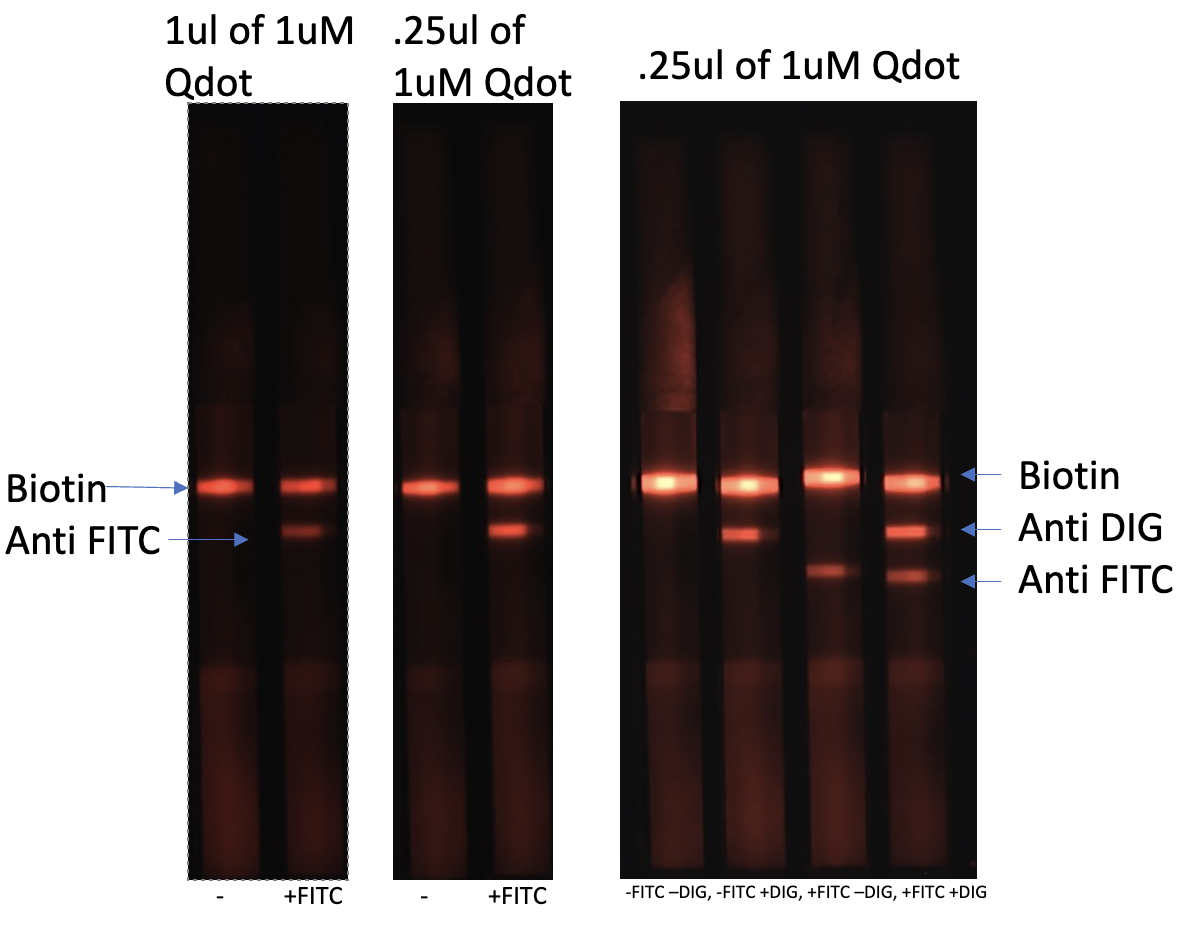
Streptavidin CdSe/ZnS Quantum Dots For Lateral Flow
$350.00- Highly stable and uniform Streptavidin CdSe/ZnS Qdots in a Ready To Use format
Streptavidin Europium Chelate Microspheres For Lateral Flow
$350.00- Ready to Use Streptavidin Europium Chelate Microspheres
- Specifically Designed Running Buffer
Streptavidin R-Phycoerythrin For Lateral Flow
$350.00- R-RPE is manufactured from a red algae
- Protein based fluorescent particles specifically desinged for lateral flow applications
- Highly absorptive fluorescent molecule with excellent detectability
RSV Antigen Assay
$200.00- Format: 25 tests per kit
- Swab Collection Method: Nasopharyngeal, Anterior Nasal, Oropharyngeal
- Results: 20 minutes
SARS-CoV-2+Influenza A+B Antigen Assay
$200.00- Format: 25 tests per kit
- Swab Collection Method: Nasopharyngeal, Anterior Nasal, Oropharyngeal
- Results: 20 minutes